Raw Material Testing
Screening of high-risk inputs such as animal products and media.

What is RAW MATERIAL TESTING and why is it important?
The regulators require that the manufacturing process for all biologics be rigorously defined and strictly controlled. Clearly, this begins with defining and controlling the input and in-process raw materials, which is crucial for cell manufacturing.
The input raw materials for a cell-based manufacturing process include the cell media and any salts, buffers, and other nutrients required for the manufacturing cells to grow and produce the biologic. While common in a research setting, the use of animal-derived materials has long been known to be of specific concern for GMP manufacturing. For example, non-irradiated bovine serum is known to present a significant risk of containing high levels of live virus. Indeed, animal-derived enzymes such as porcine trypsin carry similar viral risks to serum. Due to these significant risks, manufacturers have tried to remove the highest risk raw materials such as serum and trypsin from their cell-based production processes.
Unfortunately, it is not always possible to remove animal-derived material from the manufacturing process. Apart from a few clinical materials directly using animal donor materials such as bone or skin, some cell-based manufacturing processes may not be able to eliminate animal serum or other materials, as these are critical to production. In such cases, treatment of the high-risk material for viral reduction/removal is desirable, but unfortunately not always possible. For example, irradiation is highly effective at inactivating live virus contamination. However, it can also damage key components within the serum which are essential for the cell manufacturing process. In these cases, this animal-derived raw material must be screened to ensure that there are no significant viral risks, which is an integral part of product release testing.
Even in animal-free manufacturing processes, there is still the risk of raw materials being contaminated through environmental sources. For example, minute virus of mice (MVM or MMV) is endemic to wild mice and is shed through their feces in very high quantities. MMV has been demonstrated as the main cause of viral contamination in mAb production as it can infect and replicate in CHO cells. It is therefore easy to see how this virus can enter the cell-based production process through poor warehousing and control of the raw materials. This highlights the importance of rigorous lot release procedures and product release testing to ensure that contamination does not compromise the integrity of the biologic product.
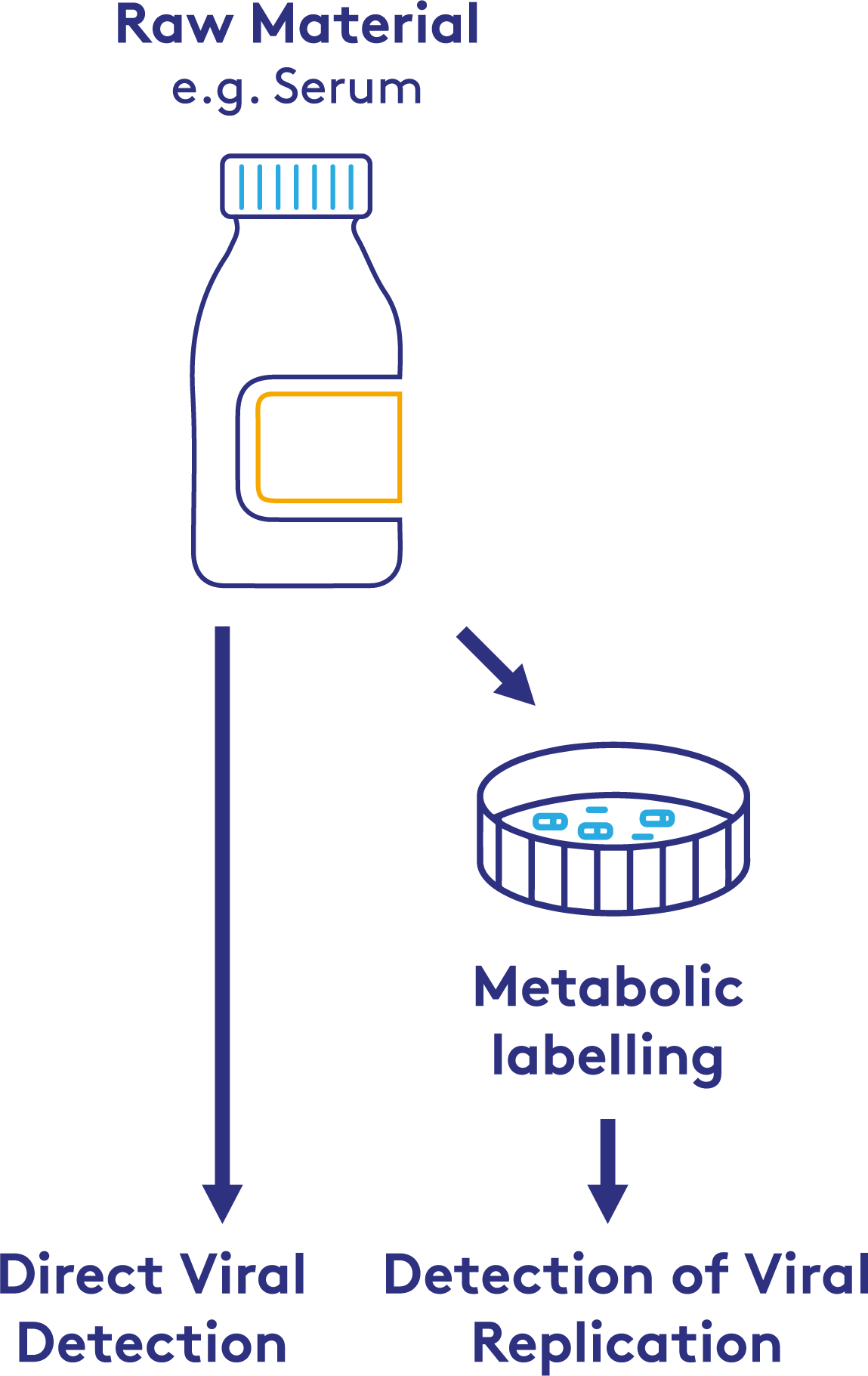
Raw materials are most often screened for viral contamination using molecular detection, specifically PCR, as it is highly sensitive and rapid. The issue with PCR is that it cannot discriminate between live-replicating virus and virus which has been inactivated through, for example, irradiation. PCR would therefore identify serum as containing virus even though it was fully inactivated, presenting no risk. More traditional cell-based methods can be used, but these can take weeks. Cell-based methods also run the risk of being permissible to infection without showing any cytopathic or histochemical indications.
Cell manufacturing processes require reliable methods to ensure the safety and integrity of raw materials. NGS can detect viral replication by identifying specific incorporation events into the viral genome. Using this technique, it is possible to discriminate between inactivated virus and virus capable of replication. This may prove advantageous to manufacturers who cannot use traditional methods to screen their raw materials, thereby enhancing the robustness of cell-based manufacturing.
Implementing NGS in product release testing can streamline the detection process, providing faster and more accurate results. This is especially critical in cell-based production, where the presence of active viral contaminants can compromise the entire manufacturing process. By integrating NGS into lot release procedures, manufacturers can ensure that only safe, uncontaminated materials are used, thus maintaining the high standards required in cell manufacturing.
Overall, using advanced techniques such as NGS for screening raw materials supports the stringent requirements of product release testing and lot release, ensuring the safety and efficacy of the final biologic products.
What we do
- mAbs and recombinant biologics
- Viral vectors
- Cell therapies
- Vaccines
- RNA based therapeutics
- Cultured meat
- Other clinical applications
Benefits of iDTECT® NGS assays for raw material testing:
✓ GMP validated
✓ Fast results – days vs weeks for cell based methods
✓ Discrimination of live versus inactivated virus
✓ Specific identification of contamination
Our Assay:
- iDTECT® Virome
SAMPLE REQUIREMENTS
The sample requirements for raw material testing will depend on the materials being tested and the approach taken. Please discuss with our PathoQuest experts who will be able to advise you.
Challenges solved
- Samples which may be difficult to test with traditional cell-based methods
- False positives from PCR detection of inactivated virus
- Identification of known and unknown contaminants instructing mitigation strategies
- Allowing manufacturing to start faster.
OTHER SERVICES
Adventitious Virus Testing
Detection of viral contamination within the manufacturing process and beyond.
READ MORE
Integration Site Analysis
Characterisation of genetic modifications for clone selection, genetic stability and lot release
Identity Confirmation
Genetic characterization of viral and plasmid products for release.
READ MORE
In Vivo Replacement
NGS as an ethical alternative to animals in biosafety testing and characterization.
READ MORE
Cell Line Characterization
Biosafety screening and stability testing of manufacturing cells.
READ MORE
HLA Genotyping
Characterizing and screening for novel and emerging cell therapies.
READ MORE
Contact us
France
+33 (0)1 70 82 17 90
Biopark -Bâtiment B,
11, rue Watt
75013 Paris, France
How can PathoQuest help?
U.S.
466 Devon Park Dr
Wayne, PA 19087
United States
France
+33 (0)1 70 82 17 90
Biopark -Bâtiment B,
11, rue Watt
75013 Paris, France
How can PathoQuest help?
Sign up for our latest news