Vaccines
PATHOQUEST SOLUTION
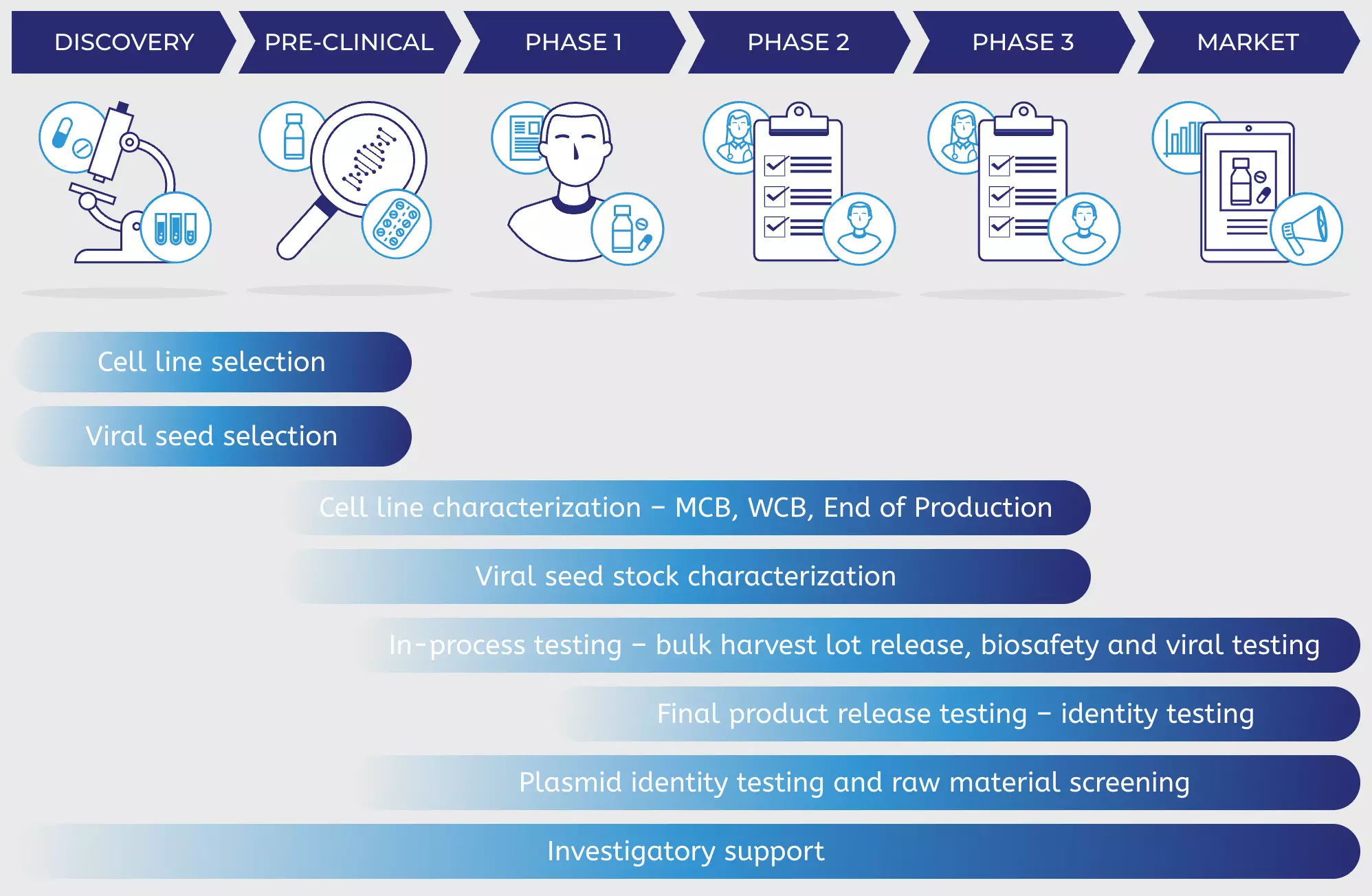
PathoQuest can help characterize, QC and release your vaccines across the manufacturing journey
DISCOVERY
PRE-CLINICAL
PHASE 1
PHASE 2
PHASE 3
MARKET
Cell line selection
Viral seed selection
Cell line characterization – MCB, WCB, End of Production
Viral seed stock characterization
In-process testing – bulk harvest lot release, biosafety and viral testing
Final product release testing – identity testing
Plasmid identity testing and raw material screening
Investigatory support
OUR EXPERTISE
PathoQuest addresses the assay compatibility and timeline challenges faced by vaccine developers and manufacturers through the application of our expert GMP NGS services. We have worked across vaccine platforms, from established recombinant and inactivated vaccine technologies to the emerging RNA and viral vector vaccine platforms. Our GMP level NGS services include vaccine testing, vaccine characterization, vaccine release, and vaccine sequencing. These services are supported by industry-leading expert bioinformatic analysis, giving you ultimate confidence in the analysis of the results.
PathoQuest actively participates in regulatory and expert industry interest groups, associations, and consortia, as well as frequently publishing in peer-reviewed journals. We lead the way in both innovation and acceptance of NGS technology in characterization and release testing across biopharmaceuticals (specifically for vaccine applications).
CHALLENGES SOLVED
PathoQuest addresses the key challenges you face in manufacturing and releasing your vaccine:
- Faster cell line and viral seed development and selection with deeper genetic insights
- De-risking your manufacturing process by rapidly screening critical raw materials such as serum and plasmids
- Reducing the cell line and viral seed characterization bottleneck, allowing you to get your vaccine to patients faster through vaccine characterization and vaccine sequencing
- Removing the need for neutralizing antibodies, which can be difficult and time-consuming to generate for vaccines
- Ensuring timely vaccine release by overcoming traditional assay compatibility issues
- Meeting corporate 3Rs ethical objectives by removing animal models
- Enhancing overall vaccine testing strategies to ensure the highest standards for safety and efficacy
Modalities
mAbs and Recombinant Proteins
Bacterial and mammalian produced proteins, hormones and peptides
READ MORE
Viral Vectors
Viral gene delivery, oncolytic and immunotherapy including manufacturing plasmids
READ MORE
Cell Therapies
Including gene modified, or unmodified stem cell therapies, allogeneic or autologous
READ MORE
Vaccines
Inactivated, live-attenuated, recombinant, RNA and viral vector products
READ MORE
RNA
Immunotherapies, antiviral, vaccines, RNAi and CRISPR based gene editing
READ MORE
Cultivated Meat
Engineered cells and tissues cultured in more ethical in vitro environments
READ MORE
Contact us
France
+33 (0)1 70 82 17 90
Biopark -Bâtiment B,
11, rue Watt
75013 Paris, France
How can PathoQuest help?
U.S.
466 Devon Park Dr
Wayne, PA 19087
United States
France
+33 (0)1 70 82 17 90
Biopark -Bâtiment B,
11, rue Watt
75013 Paris, France
How can PathoQuest help?
Sign up for our latest news