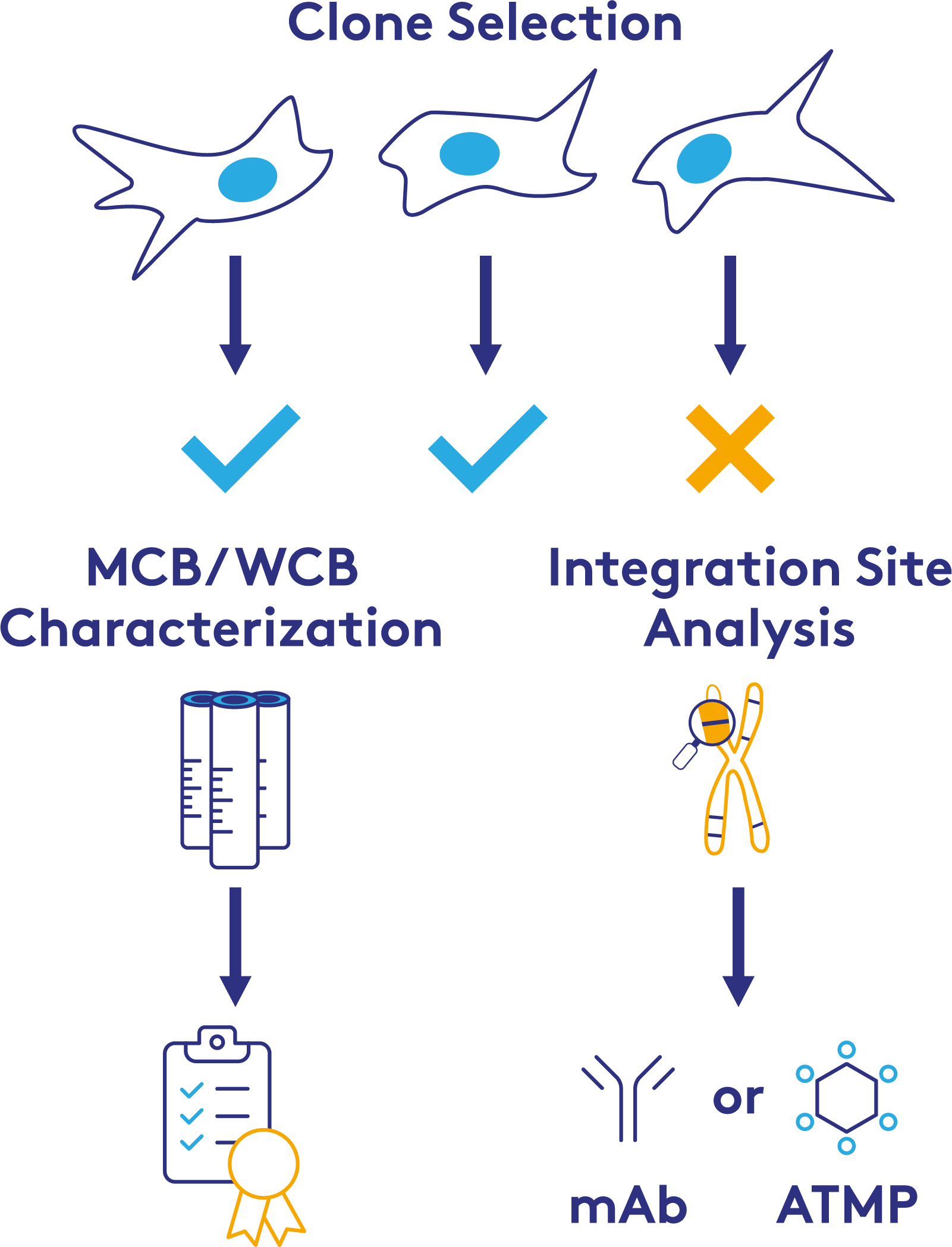
Cell Line Characterization
Biosafety screening and stability testing of manufacturing cells.


What is cell line characterization and why is it important?
According to regulatory guidance, all cells used directly as a therapy or within the manufacturing process of a biopharmaceutical must be sufficiently tested to confirm:
- Identity testing confirms the cell is as described in the submission
- Purity testing ensures that the cell does not contain any adventitious microbial or viral contaminants. It also determines if there is any contamination from other cell lines.
- Stability testing generally focuses on the genetic stability of the cell line and ensures that the cells act in the proposed manner across the expected lifetime.
Mammalian cells used for biologic production must be tested for these three categories across a simulated lifetime for the Master and Working Cell Banks (MCB/WCB), as well as the cells at the end of a production run. This ensures that any endogenous or occulted contaminating virus does not emerge unexpectedly. Genetic stability testing across this lifetime also provides assurance that the quality and yield of the biopharmaceutical product can be maintained by the manufacturer.
Classical cell line characterization packages use a range of in vitro and in vivo cell assays and other molecular assays for contamination detection. Cell line stability testing can use a mix of Sanger sequencing, PCR, and cytological methods such as FISH. A full biopharmaceutical characterization package can typically take anywhere between 3 to 6 months.
NGS-based approaches to cell line characterization developed and validated by PathoQuest offer several advantages, especially where traditional testing proves challenging. NGS can provide results much faster and is a viable alternative if there is a compatibility issue with the traditional approach (e.g., a lack of neutralizing antibody or available sample volume). NGS also offers a higher level of information, particularly in cell sequencing to characterize genetic modifications or insertions of sequences into the cell line.
Finally, using an NGS testing strategy can provide a rapid way to screen and identify favorable candidates during the cell line selection process.
What we do
Modalities Tested:
- mAbs and recombinant proteins biopharmaceuticals
- Viral vectors
- Cell therapies
- Vaccines
- RNA based therapeutics
- Cultured meat
Benefits of iDTECT® NGS assays for cell line characterization
✓ GMP Validated✓ Faster results✓ Resolves assay compatibility issues✓ No need to generate neutralizing antibodies✓ Reduces overall sample requirement for precious material✓ Specific identification of contamination✓ Meet corporate 3Rs objectives by removing animal use
Our Assays:
- iDTECT® Transcriptome
- iDTECT® Integration Site Analysis
WHY THIS APPROACH
ICH – Draft Guidline for Consultation Q5A (R2) viral safety evaluation of biotechnology products derived from cell lines of human or animal origin
FDA – Characterization and Qualification of Cell Substrates and Other Biological Materials Used in the Production of Viral Vaccines for Infectious Disease Indications
Publication:– Adventitious Virus Detection in Cells by High-Throughput Sequencing of Newly Synthesized RNAs: Unambiguous Differentiation of Cell Infection from Carryover of Viral Nucleic Acids
PathoQuest Webinar
Challenges solved
- Incompatibility of sample with traditional assays
- Very low sample volumes
- Tight clinical timelines
- Meeting corporate objectives for removing animal use
- Discriminating infectious virus from non replicating carryover
OTHER SERVICES
Adventitious Virus Testing
Detection of viral contamination within the manufacturing process and beyond.
READ MORE
Integration Site Analysis
Characterisation of genetic modifications for clone selection, genetic stability and lot release
Identity Confirmation
Genetic characterization of viral and plasmid products for release.
READ MORE
HLA Genotyping
Characterizing and screening for novel and emerging cell therapies.
READ MORE
In Vivo Replacement
NGS as an ethical alternative to animals in biosafety testing and characterization.
READ MORE
Raw Material Testing
Screening of high-risk inputs such as animal products and media.
READ MORE
Contact us
U.S.
466 Devon Park Dr
Wayne, PA 19087
United States
Sign up for our latest news
Follow Us
France
+33 (0)1 70 82 17 90
Biopark -Bâtiment B,
11, rue Watt
75013 Paris, France
Sign up for our latest news
Follow Us
How can PathoQuest help?