Substitution of in vitro and in vivo virus testing with PathoQuest NGS confirmed by French Agency
PathoQuest is pleased to announce that the Agence Nationale de Sécurité du Médicament et des Produits de Santé (ANSM) have recently published their review of PathoQuest’s NGS-based trancriptome assay for adventitious agents. CLICK HERE to view this review (in French).

The ANSM considered the latest regulatory documents on biosafety testing, including the draft revised ICH Q5A(R2) and relevant EP monographs. Their review also took into account the EU directive and the EP monograph on animal testing replacement.
The ANSM reviewed the PathoQuest transcriptome assay design and a unique head-to-head comparison with the in vivo virus test (performed in collaboration with our partners at Charles River Laboratories). For clinical trial authorizations, they concluded that the PathoQuest transcriptome assay can be used to replace:
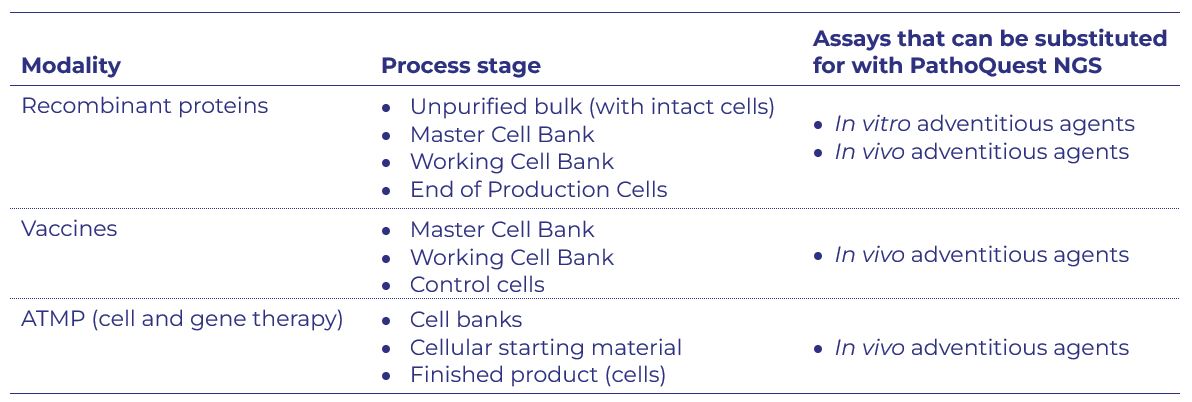
This positive outcome is another step forward as the global biologics industry adopts PathoQuest NGS-based testing in place of classical methods. Please contact us for more about how we can enable the quicker, safer and animal free QC testing of your life-saving medicines.