HLA Genotyping
Characterizing and screening for novel and emerging cell therapies.
What is HLA Genotyping and why is it important?
Human leukocyte antigen (HLA) genotype testing has traditionally been used for donor-recipient matching in organ transplantation. However, HLA genotyping can also be useful for cellular therapies as it can limit recipient response from the patient. This method can also be used for human cell line characterization and testing across biologic manufacturing as it can identify contaminating cells within the human cell line.
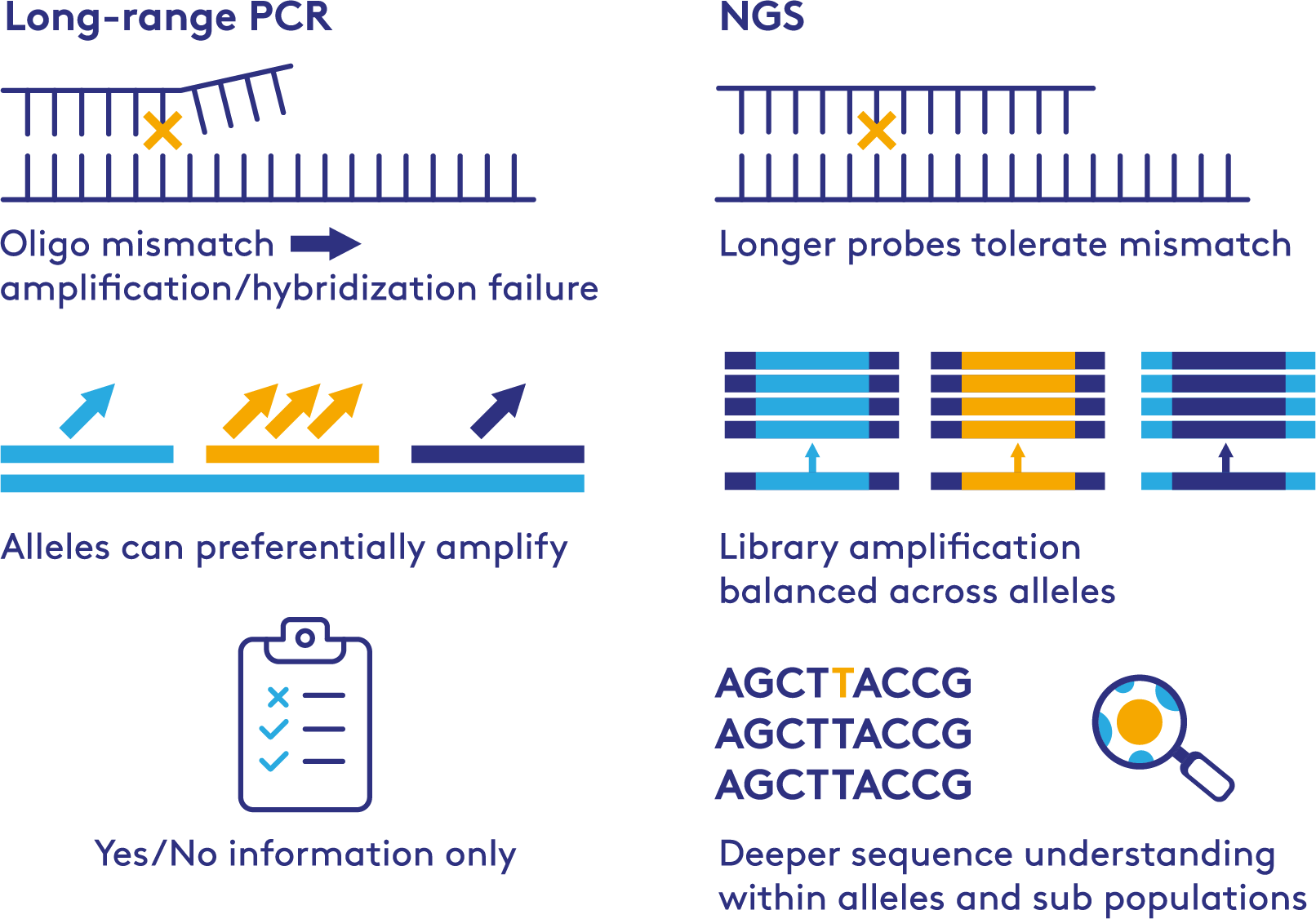
Traditional methods such as long range PCR to identify HLA specific alleles are:
✓ Fast and highly scalable✕ Can suffer from issues leading to ambiguity of results
PathoQuest uses a proven method that eliminates the issues with PCR based NGS sequencing. A hybrid capture step generates the sequencing libraries providing full coverage for all known and novel HLA alleles across 17 loci of the HLA genes.
What we do
Modalities Tested:
- Allogenic cell therapies
- Gene modified cell therapies , e.g. CAR-T
- Stem cell therapies, including mesenchymal stem cells
- Donor tissue / patient samples
- Cell lines used across biologic manufacturing
- Cell bank identity testing
Benefits of NGS for HLA genotyping
✓ Faster results
✓ ✓ Allele level resolution✓ Coverage across 17 loci, including all exons✓ Contamination detection of human cell lines
PCR VS NGS for HLA Testing
WHY THIS APPROACH
Publication: The Importance of HLA Assessment in “Off-the-Shelf” Allogeneic Mesenchymal Stem Cells Based-Therapies
Publication: Reduced PCR-generated errors from a hybrid capture-based NGS assay for HLA typing
Publication: Regulatory considerations for developing a phase I investigational new drug application for autologous induced pluripotent stem cells‐based therapy product
PathoQuest HLA Genotyping Flyer
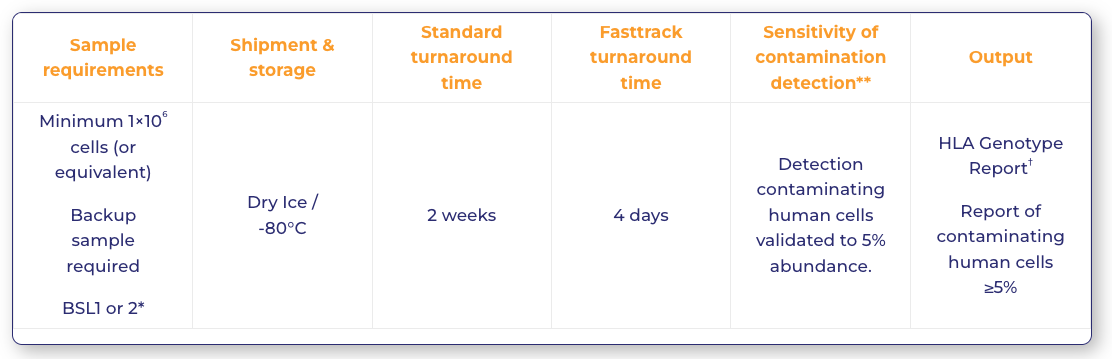
Sample requirements
|
Sample requirements |
Shipment & storage | Standard turnaround time | Fasttrack turnaround time | Sensitivity of contamination detection** |
Output |
| Minimum 1×106 cells (or equivalent)
Backup sample required BSL1 or 2* |
Dry Ice / -80°C |
2 weeks | 4 days | Detection contaminating human cells validated to 5% abundance. | HLA Genotype Report†
Report of contaminating human cells ≥5% |
*Biosafety level classifications can vary between regulatory authorities – contact PathoQuest to discuss.
** NGS should be considered as semi-quantitative in this application. Abundance figures are provided at the occurrence rate of the contaminating allele as it appears within the read data. Detection of contaminants may be possible below the validated 5% level. Please discuss with our experts if this is required.
† Genotyping of genes: HLA-A/-B/-C/-E/-F/-G/-H, DRB1, DRB3/4/5, DQA1, DQB1, DPA1, DPB1, MICA and MICB
*Biosafety level classifications can vary between regulatory authorities – contact PathoQuest to discuss.
** NGS should be considered as semi-quantitative in this application. Abundance figures are provided at the occurrence rate of the contaminating allele as it appears within the read data. Detection of contaminants may be possible below the validated 5% level. Please discuss with our experts if this is required.
† Genotyping of genes: HLA-A/-B/-C/-E/-F/-G/-H, DRB1, DRB3/4/5, DQA1, DQB1, DPA1, DPB1, MICA and MICB
Challenges solved
- PCR failure due to primer mismatch
- Balanced amplification across all HLA alleles
- Base level resolution, including sub population detection
- Detection of contaminating human cells within a human cell line
OTHER SERVICES
Adventitious Virus Testing
Detection of viral contamination within the manufacturing process and beyond.
READ MORE
Integration Site Analysis
Characterisation of genetic modifications for clone selection, genetic stability and lot release
Identity Confirmation
Genetic characterization of viral and plasmid products for release.
READ MORE
Cell Line Characterization
Biosafety screening and stability testing of manufacturing cells.
READ MORE
In Vivo Replacement
NGS as an ethical alternative to animals in biosafety testing and characterization.
READ MORE
Raw Material Testing
Screening of high-risk inputs such as animal products and media.
READ MORE
Contact us
U.S.
466 Devon Park Dr
Wayne, PA 19087
United States
Sign up for our latest news
Follow Us
France
+33 (0)1 70 82 17 90
Biopark -Bâtiment B,
11, rue Watt
75013 Paris, France
Sign up for our latest news
Follow Us
How can PathoQuest help?