Viral Vectors
CUSTOMER CHALLENGE
Viral vectors can be used for a range of applications, from direct in vivo gene delivery in genetic therapies to in vitro transduction for gene-modified cell therapies such as CAR-T, as well as oncolytic applications. Manufacturing the virus is almost always highly complex, involving critical input materials such as plasmids, meaning there are much higher production costs than traditional biopharmaceuticals.
Viral vector characterization is essential to ensure the quality and efficacy of these therapies. Traditional testing approaches, such as in vitro virus testing, often do not provide the requisite detail of information that the regulators require. Combined with widespread compatibility and volume requirement issues, viral safety testing becomes crucial to ensure safety and regulatory compliance. Furthermore, plasmid screening is necessary to identify and verify the plasmids used in the production process. Customers must find alternative testing and release strategies, including comprehensive viral vector services, for these incredible new modalities.
PATHOQUEST SOLUTION
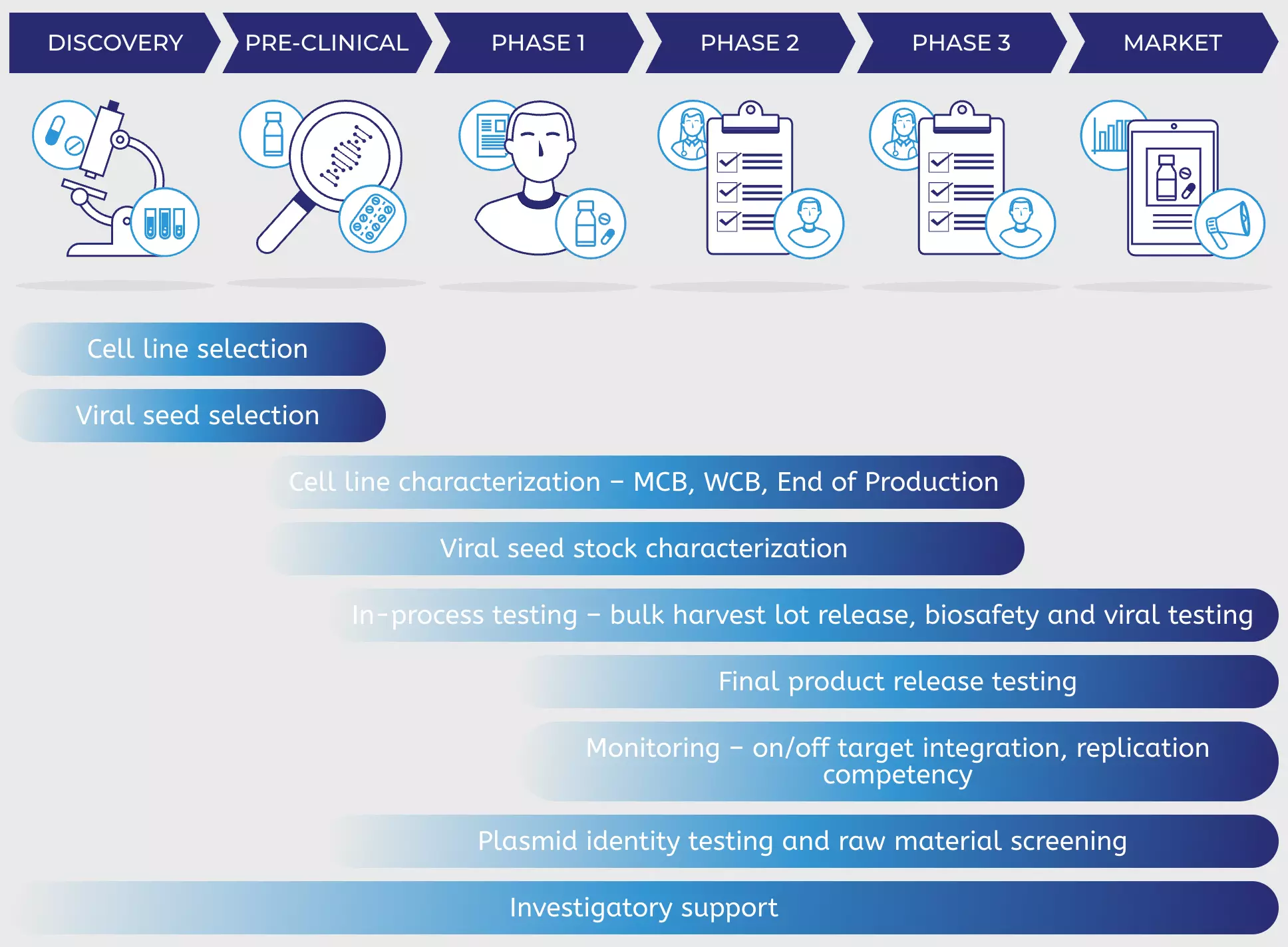
PathoQuest can help characterize, QC and release your viral vectors across the manufacturing journey
DISCOVERY
PRE-CLINICAL
PHASE 1
PHASE 2
PHASE 3
MARKET
Cell line selection
Viral seed selection
Cell line characterization – MCB, WCB, End of Production
Viral seed stock characterization
In-process testing – bulk harvest lot release, biosafety and viral testing
Final product release testing
Monitoring – on/off target integration, replication competency
Plasmid identity testing and raw material screening
Investigatory support
OUR EXPERTISE
PathoQuest has been working with many leading global innovators to help them address the unique challenges that viral vectors present. By using our expert GMP NGS services, our clients have been able to overcome compatibility issues with traditional cell or animal-based testing and characterization, such as in vitro virus testing and viral vector characterization, enabling them to deliver life-changing therapies to patients. We also offer comprehensive viral vector services, including viral safety testing and plasmid screening, to ensure the safety of these therapies.
PathoQuest actively participates in regulatory and expert industry interest groups, associations, and consortia, as well as frequently publishing in peer-reviewed journals. We lead the way in both innovation and acceptance of NGS technology, especially when applied to the specific challenges of viral vector QC.
CHALLENGES SOLVED
PathoQuest addresses the key challenges you face in manufacturing and releasing your viral vector by addressing key concerns:
- Faster cell line development and clone selection with deeper genetic insights
- De-risking your manufacturing process by rapidly screening critical raw materials such as serum and plasmids
- Reducing the cell line and plasmid screening bottleneck, allowing you to get your therapy into clinic faster
- Detection of sequence variation to low levels, ensuring the highest possible quality and safety
- Addressing key concerns with viral vector characterization and in vitro virus testing by reducing volume requirements and cell-based assay compatibility issues
- Providing additional assurance of biosafety where processes such as viral clearance testing can be challenging
- Meeting corporate 3Rs ethical objectives by removing animal models
Modalities
mAbs and Recombinant Proteins
Bacterial and mammalian produced proteins, hormones and peptides
READ MORE
Viral Vectors
Viral gene delivery, oncolytic and immunotherapy including manufacturing plasmids
READ MORE
Cell Therapies
Including gene modified, or unmodified stem cell therapies, allogeneic or autologous
READ MORE
Vaccines
Inactivated, live-attenuated, recombinant, RNA and viral vector products
READ MORE
RNA
Immunotherapies, antiviral, vaccines, RNAi and CRISPR based gene editing
READ MORE
Cultivated Meat
Engineered cells and tissues cultured in more ethical in vitro environments
READ MORE
Contact us
France
+33 (0)1 70 82 17 90
Biopark -Bâtiment B,
11, rue Watt
75013 Paris, France
How can PathoQuest help?
U.S.
466 Devon Park Dr
Wayne, PA 19087
United States
France
+33 (0)1 70 82 17 90
Biopark -Bâtiment B,
11, rue Watt
75013 Paris, France
How can PathoQuest help?
Sign up for our latest news