Mission
The PathoQuest mission is to develop proprietary, rapid turn-around-time GMP Next Generation Sequencing (NGS) assays and advanced bioinformatic approaches designed to accelerate adventitious agent detection and bioprocess characterization. Our innovative solutions serve as an ethical alternative to traditional methods by aiming to replace animal testing, ensuring the safe and high-quality production of biopharmaceuticals.
Modalities
mAbs and Recombinant Proteins
Bacterial and mammalian produced proteins, hormones and peptides
READ MORE
Viral Vectors
Viral gene delivery, oncolytic and immunotherapy including manufacturing plasmids
READ MORE
Cell Therapies
Including gene modified, or unmodified stem cell therapies, allogeneic or autologous
READ MORE
Vaccines
Inactivated, live-attenuated, recombinant, RNA and viral vector products
READ MORE
RNA
Immunotherapies, antiviral, vaccines, RNAi and CRISPR based gene editing
READ MORE
Cultivated Meat
Engineered cells and tissues cultured in more ethical in vitro environments
READ MORE
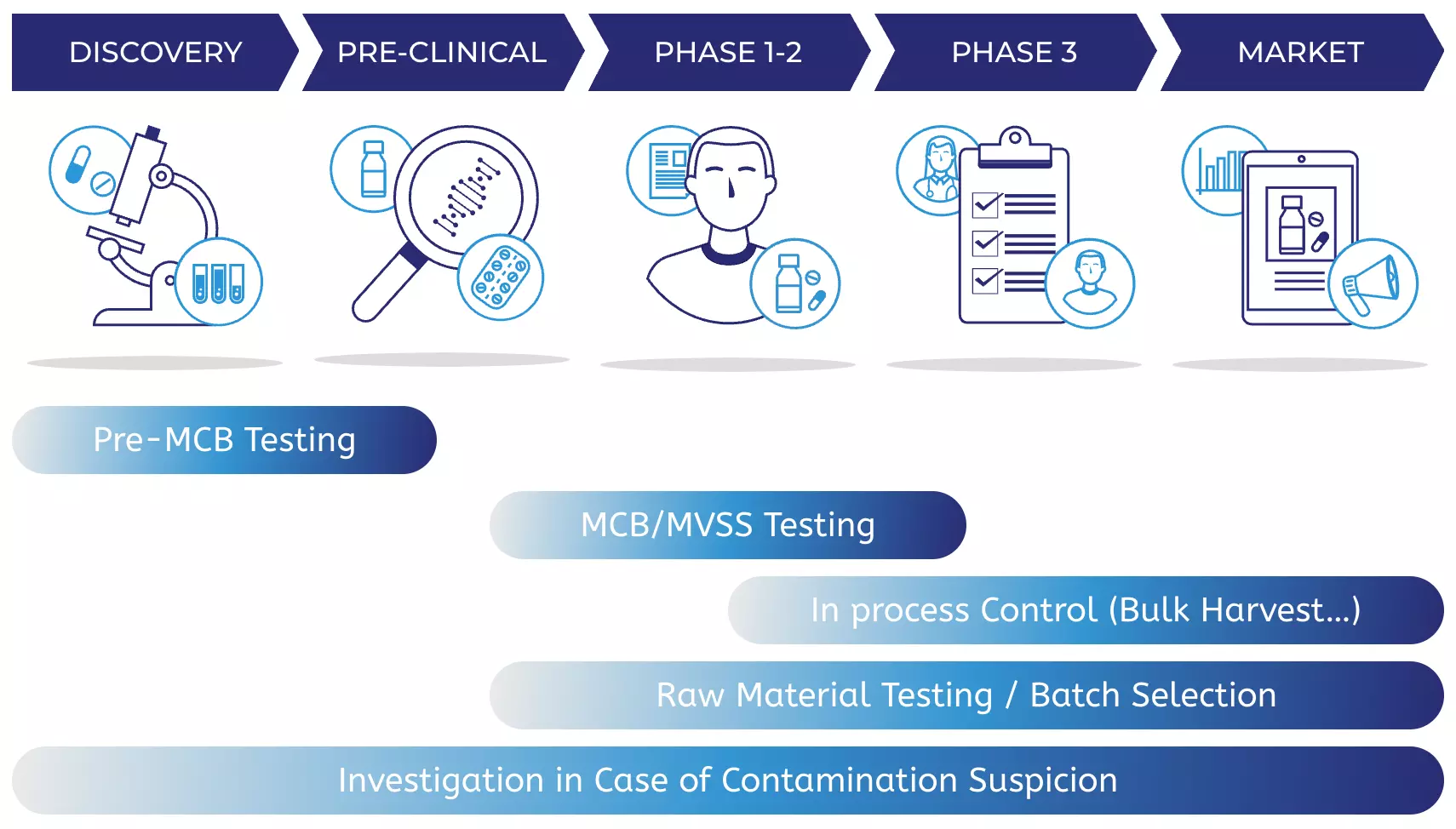
When we can help
From pre-clinical development to commercial product. Our biosafety testing and characterization services can help you get your product to patients faster.
DISCOVERY
PRE-CLINICAL
PHASE 1-2
PHASE 3
MARKET
Pre-MCB Testing
MCB/MVSS Testing
In process Control (Bulk Harvest…)
Raw Material Testing / Batch Selection
Investigation in Case of Contamination Suspicion
3Rs
Next Generation Sequencing (NGS) is the ethical alternative to in vivo testing for the quality control testing of biopharmaceuticals.
PathoQuest, in partnership with Charles River Laboratories, is the first Contract Research Organization (CRO) to conclusively demonstrate the superiority of NGS-based biosafety testing over conventional in vivo testing methods for biosafety testing and biopharma characterization.

3Rs
Next generation sequencing is the ethical alternative to in vivo testing for the quality control testing of biopharmaceuticals.
PathoQuest, in partnership with Charles River Laboratories, is the first CRO to conclusively demonstrate the superiority of NGS over conventional in vivo biosafety testing and characterization.

Contact us
France
+33 (0)1 70 82 17 90
Biopark -Bâtiment B,
11, rue Watt
75013 Paris, France
How can PathoQuest help?